LGSOC and STAAR News
As the leading LGSOC patient advocacy organization, we also keep our community informed with the newest research updates, treatment news, and advancements shaping the future of care.

In the News

12/4/2023: STAAR honored for Inspirational Collaborations that raised the Voices of LGSOC
STAAR Ovarian Cancer Foundation was honored Dec. 1 with an Inspirational Collaboration Award during the third annual World Ovarian Cancer Coalition Impact Awards.
The award celebrates the achievements of organizations that worked in partnership to make a difference in ovarian cancer. STAAR was recognized for its work in two collaborations that brought low-grade serous ovarian cancer into “sharp focus,” said Tristan Bilash, patient advocate and advisor to the World Ovarian Cancer Coalition, who presented the award.
“The first was a multi-stakeholder global survey of those living with a diagnosis of this type, aimed at better understanding the people and circumstances behind the diagnosis. The second major collaboration brought the patient voice to a United States Food and Drug Administration meeting they arranged, and where they met with U.S. officials, researchers, drug companies, and industry. This meeting was the first externally led patient-focused drug development meeting for any type of ovarian cancer, which has the potential of changing the future of drug development and approvals for this disease,” Bilash said.
Nicole Andrews, Chair of STAAR Ovarian Cancer Foundation, said: “Working in a rare cancer space with little funding, patients spread across the globe, and fewer researchers has allowed our board to understand the importance of collaboration for higher impact. This year‘s work helping low-grade serous cancer patients have their voices heard through the international patient needs survey and the Voices of LGSOC event would not have been possible without our global collaboration partners with whom we share this award: Cure Our Ovarian Cancer, World Ovarian Cancer Coalition, and Drs. Gershenson, Sun, Gourley, Grisham, Banerjee, Curry and deFazio—and of course Verastem Oncology, who generously sponsored these projects. Thank you to the World Ovarian Cancer Coalition for their invaluable contributions in promoting global collaborations.”
Clara MacKay, CEO of the World Ovarian Cancer Coalition said: “With cases set to rise by almost 42% by 2040, the work of advocates who work on behalf of all of those impacted by ovarian cancer is more important than ever. It is truly humbling to see such incredible work achieved over the last year by all of our winners, nominees, and the wider ovarian cancer community.”
About the World Ovarian Cancer Coalition
The World Ovarian Cancer Coalition is a not-for-profit organization, formally established in 2016, working across the globe towards a world where everyone with ovarian cancer has the best chance of survival, and the best quality of life—wherever they may live. More information can be found on www.worldovariancancercoalition.org
About STAAR Ovarian Cancer Foundation
STAAR Ovarian Cancer Foundation is the only nonprofit in the United States dedicated to low-grade serous ovarian cancer. It was co-founded by three women with LGSOC in early 2020. The foundation works with the global charity Cure Our Ovarian Cancer to advance research opportunities in the United States to find better treatment options for LGSOC.
For more information contact:
Phaedra Charlton
Director of Communications and Marketing
World Ovarian Cancer Coalition
phaedra@worldovariancancercoalition.org
Nicole Andrews
Board Chair
STAAR Ovarian Cancer Foundation
Nicole@staaroc.org
World Ovarian Cancer Coalition Impact Awards
3/11/2024:
Orphan Drug Designation for Low-Grade Serous Ovarian Cancer is Beacon of Hope
The U.S. Food and Drug Administration has granted an orphan drug designation to a low-grade serous ovarian cancer treatment currently in clinical trials—a huge step forward for treating a rare cancer with a high rate of recurrence.
Verastem Oncology received the orphan drug designation for the drug avutometinib alone or in combination with defactinib for the treatment of patients with recurrent low-grade serous ovarian cancer.
LGSOC is a distinct ovarian cancer, making up 5-10% of all ovarian cancer diagnoses. While the cancer cells are slower growing than the more common high-grade serous ovarian cancer, LGSOC tends to be resistant to chemotherapy, leading to poor outcomes. It also disproportionately affects younger women, with an average age at diagnosis of 45.
Diagnosis is often delayed because LGSOC symptoms can be confused with other illnesses. Symptoms include bloating, feeling full quickly, fatigue, indigestion, menstrual irregularities, changes in bowel habits and painful intercourse.
VOICES OF LGSOC
The FDA’s orphan drug designation comes on the heels of the first-ever externally led patient focused drug development meeting that STAAR Ovarian Cancer hosted last fall. The meeting amplified the voices of patients with LGSOC and conveyed to the FDA that this is a distinct disease in need of specialized treatment.
“Our stories, experiences and advocacy during the meeting served as a powerful catalyst,” said STAAR Board Chair Nicole Andrews. “It brought attention to the urgent need for more research, better treatments, and a deeper understanding of the challenges faced by those with low-grade serous ovarian cancer. Verastem Oncology's orphan drug designation is a direct example of our collective effort, showcasing the real-world impact of patient advocacy.”
ORPHAN DISEASES
Orphan drug designations are granted to drugs targeting rare diseases with unmet medical needs—such as LGSOC—providing incentives to pharmaceutical companies, including extended market exclusivity and financial incentives, encouraging investment in research and development for rare diseases.
“The FDA Orphan Drug Designation for avutometinib alone or in combination with defactinib in low-grade serous ovarian cancer is an important step in recognizing this rare cancer as a distinct disease that currently has no FDA-approved treatments,” said Dan Paterson, president and chief executive officer of Verastem Oncology. “We are rapidly advancing the development program for avutometinib and defactinib in low-grade serous ovarian cancer with our ongoing Phase 3 clinical trial to deliver this new combination treatment to patients as quickly as possible. We remain on track to begin submission of an NDA to the FDA for Accelerated Approval of this combination in the first half of 2024 and preparing for a potential launch in 2025.”
An orphan disease is a rare disease or condition that affects fewer than 200,000 people in the United States and are serious or life threatening. In 1983, the U.S. government passed the Orphan Drug Act to give drug companies financial benefits for developing orphan drugs. This law is meant to help bring more drugs to patients with rare diseases.
“This orphan drug designation is a beacon of hope for those with low-grade serous ovarian cancer,” Andrews said. “It encourages pharmaceutical companies to invest in groundbreaking treatments, ultimately transforming the landscape for patients who have long been underserved.”
Advantages of Orphan Drug Designations for LGSOC:
1. Targeted Therapies: Orphan drug designations facilitate the development of targeted therapies tailored to the unique molecular and genetic characteristics of LGSOC, enhancing treatment precision and efficacy.
2. Accelerated Approval Process: The orphan drug designation expedites the regulatory approval process, allowing promising treatments for LGSOC to reach patients more swiftly, addressing critical unmet needs in a timely manner.
3. Research Investment: By incentivizing pharmaceutical companies to invest in LGSOC research, orphan drug designations foster innovation and the exploration of novel treatment modalities, ultimately expanding the therapeutic armamentarium against this rare disease.
4. Patient Access: Orphan drug designations enhance patient access to innovative treatments by facilitating affordability and availability, ensuring that individuals afflicted with LGSOC have equitable access to potentially life-saving therapies.
STAAR Ovarian Cancer Foundation is the only U.S.-based nonprofit dedicated to low-grade serous ovarian cancer. It was co-founded by three women with LGSOC in early 2020.
4/7/2024: STAAR-funded endocrine study published in British Journal of Cancer
The first low-grade serous ovarian cancer research to receive funding from STAAR Ovarian Cancer Foundation has been published in the British Journal of Cancer.
STAAR partnered with Cure Our Ovarian Cancer to contribute $70,000 to research by Dr. KK Wong of University of Texas MD Anderson Cancer Center. The research investigated estrogen signaling in low-grade serous ovarian cancer (LGSOC) to better understand how the cancer cells use estrogen.
“This is a unique opportunity to study the complex and elusive estrogen receptor signaling pathway in low-grade serous ovarian cancer, which would hopefully result in a successful and improved anti-hormone therapy,” Wong said.
FINDINGS
The paper, The Prognostic Value of MEK-pathway associated estrogen receptor signaling activity for female cancers, looked at the estrogen receptor (ER) signaling pathway activities of breast, ovarian, endometrial, and cervical cancers to identify which may predict endocrine therapy responsiveness.
Because endocrine therapy has been shown to be effective against breast cancer cells, but not reliably against other gynecologic cancers, Wong's research sought to understand the role of ER signaling activity in the development of gynecologic cancers by looking at the difference of the pathway activation between normal and tumor tissues.
The research found that ER signaling is prognostic for gynecological cancers and that MEK pathway activity is associated with ER signaling in patients with the gynecologic cancers. Targeting both the estrogen receptor and MEK pathways (a chain of proteins associated with cell growth) may aid the development of endocrine therapy strategies.
IMPLICATIONS FOR LOW-GRADE SEROUS OVARIAN CANCER
"We found that our approach can better predict the response of patients to endocrine therapy, including [those with] LGSOC," Wong said. "In addition, we identified a few genes which are associated with endocrine therapy resistance."
Previous research has shown that hormonal maintenance therapy--such as aromatase inhibitors Letrozole, Anastrozole, and Exemestane—can result in a lower risk of progression in patients with stage II-IV low-grade ovarian serous carcinoma.
The paper also discusses some previous studies into endocrine therapy for ovarian cancer:
"Letrozole has been suggested to be valuable as a maintenance treatment of high-grade serous ovarian cancer, especially in patients with chemoresistance or residual disease. Another retrospective study indicates that endocrine therapy could be a practical strategy to postpone subsequent chemotherapy for relapsed high-grade serous ovarian cancer. From a phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors, partial responses were only observed in 14% patients."
"It is important to identify patients who will benefit the most from endocrine therapy and avoid unnecessary treatment for patients who will not respond," Wong said.
Wong also is researching patient resistance to the MEK inhibitor trametinib in another STAAR-funded study.
LGSOC has fewer treatment options than the more common high-grade serous ovarian cancer. It also tends to be diagnosed in younger patients, with an average age at diagnosis of 45, compared to 62 people for high-grade. Because the cancer cells are slower-growing than high-grade cells, they tend to be resistant to chemotherapy. The average prognosis for LGSOC is about 9 years.
STAAR Ovarian Cancer Foundation is the only U.S.-based nonprofit dedicated to low-grade serous ovarian cancer. It has contributed more than $500,000 to research low-grade serous ovarian cancer since its founding by three women with LGSOC in 2020.
12/20/2024: STAAR to host first LGSOC Patient Conference
STAAR Low-Grade Serous Ovarian Cancer Foundation is bringing LGSOC patients together for the first time for a conference to build community and focus on the specific challenges and latest research developments for this rare cancer.
The two day conference will be held February 7-8, 2025, in Houston, Texas, and will include a visit to the research laboratories at the University of Texas, MD Anderson Cancer Center.
What is STAAR?
STAAR was founded in 2020 when three women facing low-grade serous ovarian cancer recognized the urgent need for dedicated funding and support—a need unmet by the larger charities. The foundation exists to fund research for better treatment options for low-grade serous carcinoma, a rare (about 5% of ovarian cancer cases) and extremely underfunded ovarian cancer.
Conference Mission and Vision
Mission: To empower patients, survivors, and caregivers by providing a supportive space to share experiences, access the latest medical information, and connect with resources that enhance their quality of life.
Vision: To create a dynamic, informative, and inclusive gathering where attendees leave with a deeper understanding of low-grade ovarian cancer, empowered to make informed decisions about their care, and inspired by a community dedicated to advocacy, research, and support.
Sessions include:
-
State of LGSOC by Dr. David Gershenson, University of Texas MD Anderson Cancer Center
-
Side Effect Management
-
LGSOC Research Timeline: What is on the horizon? Dr. Brian Slomovitz, Mount Sinai Medical Center
-
Sexual Health by Dr. Leslie Schover, psychologist, author, former faculty at University of Texas MD Anderson Cancer Center
-
Self-Care for Caregivers: Preventing Burnout by Danielle Peterson, Ovarian Cancer Research Alliance
-
Understanding Clinical Trials by Dr. Rachel Grisham, Memorial Sloan Kettering Cancer Center
-
Thriving While Surviving by Danielle Peterson, Ovarian Cancer Research Alliance
-
Understanding Recurrence by Dr. Lauren Cobb, University of Texas, MD Anderson Cancer Center
-
Understanding the Pharma Development Process and the Goal for LGSOC medicine
-
The Science and Strategies of Nutrition during Treatment by Erin Pellegrin, Unite for Her
Keynote speakers
Dr. David Gershenson is an unparalleled authority in the field of low-grade serous ovarian cancer with more than 47 years of dedicated experience at The University of Texas MD Anderson Cancer Center. His profound insights into LGSOC are the result of extensive clinical and translational research, making him the foremost authority in this field. With more than 450 peer-reviewed articles, 188 invited articles, book chapters, and editorials, Dr. Gershenson’s impact on LGSOC research is immeasurable. He served as Editor-In-Chief of the esteemed journal Gynecologic Oncology for 18 years and continues to contribute as Editor Emeritus.
Dr. Rachel Grisham is an Associate Attending within the Department of Medicine at Memorial Sloan Kettering Cancer Center and Associate Professor of Medicine at Weill Cornell Medical College. She is board certified in Medical Oncology and is the Section Head of Ovarian Cancer Treatment and the Director of Gynecologic Medical Oncology at MSKCC Westchester. Dr. Grisham’s research focuses on determining the molecular drivers of low-grade serous ovarian cancer and the development of targeted treatment strategies for patients with recurrent ovarian cancer. She has served as the principal investigator for more than 25 clinical trials and serves on multiple committees within the American Society of Clinical Oncology, NRG Oncology, and Society of Gynecologic Oncology. She is the recipient of an ASCO Merit Award and a Conquer Cancer Career Development Award, as well as numerous other grants and awards
2/18/2025: STAAR Low-Grade Serous Ovarian Cancer Celebrates 5 Years with First-Ever Patient Conference

February marks the 5th anniversary of STAAR Low-Grade Serous Ovarian Cancer. We celebrated the milestone with the first-ever Patient Conference in Houston, bringing together patients, caregivers, medical professionals and advocates.
The conference provided an opportunity for the LGSOC community to connect, share experiences, and gain insights into the latest research and treatment options for low-grade serous ovarian cancer.
Highlights:
-
Informative Presentations: Leading experts in the field delivered presentations on a wide range of topics, including advancements in surgical techniques, emerging therapies, and clinical trials.
-
Patient-Led Workshops: Patients shared their personal stories and offered practical advice on managing the physical and emotional challenges of living with low-grade serous ovarian cancer.
-
Caregiver Support Sessions: Caregivers had the opportunity to connect with one another, share their experiences, and learn strategies for providing support to their loved ones.
-
Wellness Activities: “Find a new hobby” sessions provided attendees with tools for relaxation and stress reduction through activities like water coloring, crocheting, mahjong, pickleball, needlepointing and knitting.
-
Celebratory Gala: We closed out the weekend with a gala dinner, honoring the STAAR Foundation's achievements and recognizing the strength and resilience of the low-grade serous ovarian cancer community.
Legacy of Hope
Founded in 2020, STAAR has advocated for patients and families affected by this rare and often under-recognized disease. Our mission is to raise awareness, fund research, and support everyone impacted by low-grade serous ovarian cancer.
Over the past five years, STAAR has made significant strides in advancing research, improving patient care, and fostering a sense of community among people affected by low-grade serous ovarian cancer. The STAAR Patient Conference was a testament to our commitment to our mission and a celebration of the progress we’ve made.
Looking Ahead
As the STAAR Low-Grade Serous Ovarian Cancer Foundation enters our next chapter, we remain dedicated to our mission to improve the lives of people affected by low-grade serous ovarian cancer. The success of our first Patient Conference shows the power of our community and the progress we’ve made in advocacy and advancing research.
With Gratitude
STAAR is an entirely volunteer-run organization, dedicated to advancing research into treatments for LGSOC. We could not have hosted this conference without the generous support of sponsors including Verastem Oncology, Bristol Myers Squibb and Not These Ovaries.













4/7/2024: STAAR-funded endocrine study published in British Journal of Cancer
Latest Research Expands on Efficacy of FDA-Approved LGSOC Treatment
7/22/2025
The latest research published in the Journal of Clinical Oncology strengthens the promise of the first-ever treatment developed for low-grade serous ovarian cancer (LGSOC). The findings expand the potential reach of the drug combination avutometinib and defactinib, bringing hope to more people facing this historically hard-to-treat disease.
FDA Approval & Expanding Options for LGSOC
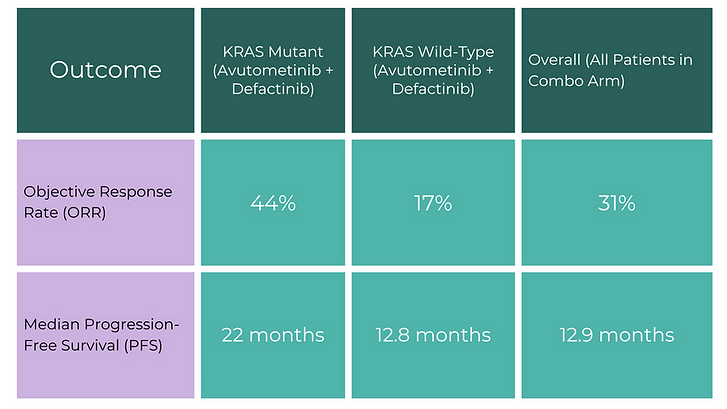
In May, the U.S. Food and Drug Administration (FDA) approved the drug combination avutometinib (a RAF/MEK inhibitor) and defactinib (a FAK inhibitor) for the treatment of recurrent LGSOC in patients with a KRAS mutation. Now, new results from the phase II RAMP 201 clinical trial reveal the duo may also benefit patients without the KRAS mutation—known as KRAS “wild type.”
Key Findings from the RAMP 201 Study
Researchers found significant improvements in both objective response rate (ORR) and progression-free survival (PFS) for patients treated with avutometinib and defactinib—regardless of KRAS status:
The combination therapy substantially improved clinical outcomes — 82% of all patients saw at least some reduction in target lesions, even if they didn't all meet the criteria for objective response.
"The fact that a majority of patients had some reduction in target lesions, regardless of KRAS mutation status, underscores the advancement the combination represents in this rare ovarian cancer and its potential to be the new standard of care in recurrent low-grade serous ovarian cancer."
— Professor Susana Banerjee, Global Lead Principal Investigator in RAMP 201, Consultant Medical Oncologist, The Royal Marsden NHS Foundation Trust
Why This Matters: Traditional Treatments Fall Short
LGSOC is rare—fewer than 10% of all ovarian cancers—and typically affects younger women, with an average diagnosis age of 45. Unlike high-grade serous ovarian cancer, LGSOC generally grows slowly but can be resistant to standard chemotherapy.
Standard frontline treatment includes surgery and chemotherapy, often followed by hormone therapy. But in recurrence, traditional chemotherapy yields only a 13% response rate. In contrast, this new drug combination produced robust tumor shrinkage in nearly a third of patients and extended time before progression.
Safety: A Well-Tolerated, Lower-Toxicity Option
The RAMP 201 trial showed the avutometinib/defactinib combo to be generally well-tolerated. The common side effects included fatigue, swelling, rashes, nausea, vomiting, and diarrhea—typical for MEK and FAK inhibitors—with just 10% of patients discontinuing due to side effects. This is a promising, less toxic alternative to conventional chemotherapy.
The Future: Toward a New Standard of Care
With a phase III trial already underway to evaluate the combination in patients with and without a KRAS mutation, avutometinib and defactinib are pushing the boundaries in LGSOC treatment—bringing new hope to patients who haven’t responded to previous therapies.
Supporting Progress: STAAR Low-Grade Serous Ovarian Cancer Foundation
Progress in LGSOC research wouldn’t be possible without advocacy and funding. The STAAR Low-Grade Serous Ovarian Cancer Foundation is the first U.S. nonprofit dedicated exclusively to low-grade serous ovarian cancer. Since 2020, when it was started by three women with LGSOC, STAAR has helped fund more than $1 million into advancing research targeted at this rare cancer.
To read more, get involved, or support ongoing LGSOC research, visit staaroc.org